
Our Research
Seq Biomarque is Developing Alzheimer’s Molecular Blood-Based Biomarker Tests
The Seq Biomarque scientific team is working with guidance from worldwide leaders in Alzheimer’s Disease (AD) clinical research to develop a non-invasive (blood-based), highly sensitive (single-digit pg/mL), and cost-effective multi-factor AD diagnostic test.
Novel Biomarker Focus
While Seq Biomarque is developing biomarker tests for traditional markers of AD pathology (Tau and Beta-Amyloid), Seq Biomarque is also developing novel blood-based biomarkers that are directly relevant to the diagnostic AD continuum.
There is currently an unmet need for biomarkers that are associated with early detection and the preclinical stages of AD Early diagnosis which will allow for the most optimal (therapeutic) AD patient care. Therefore, Seq Biomarque is particularly interested in pathology-based biomarkers that are associated with early detection and the preclinical stages of AD.
Seq Biomarque is also interested in mid- to late-stage AD biomarkers that may be useful for monitoring therapeutic efficacy and treatment effects.
Seq Biomarque believes that a multifactor AD biomarker panel may serve as a useful diagnosis tool that may provide additional information that complements existing clinical AD assessments and neuro-imagining tests.
Seq Biomarque’s test platform is designed to be easily adapted to allow for the inclusion of new biomarkers.
Seq Biomarque’s test platform is expandable to allow for the high-throughput simultaneous testing of multiple independent specimens.
Hallmark of Alzheimer’s Disease: The “Tau” Protein
- The “Tau” protein is well established as a microtubule-associated protein in neurons (Tau aids in the stabilization of other proteins known as microtubules).
- Abnormal Tau processing is observed in Alzheimer’s disease brains and is considered a hallmark of AD pathology.
- Abnormal Tau processing includes a modification known as phosphorylation.
- Abnormal Tau phosphorylation may disrupt the normal function of the protein and ultimately contribute to Alzheimer’s disease
- Tau contains 79 potential phosphorylation sites, and the phosphorylation of Tau at specific sites may be pathologically and diagnostically relevant.

Why “Tau” as a Biomarker?
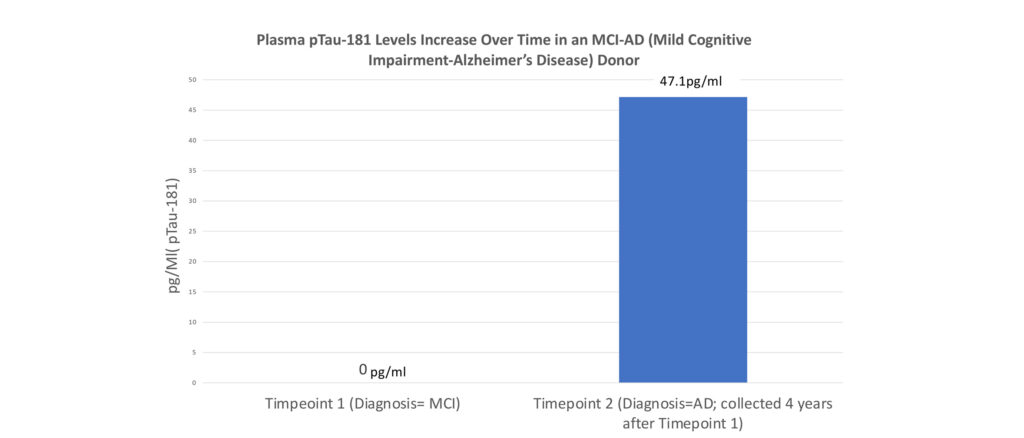
Tau can cross the blood-brain barrier, and therefore, brain-derived Tau is detectable in the blood. Previous studies indicate that plasma levels of phosphorylated Tau are higher in AD patients compared to normal controls.
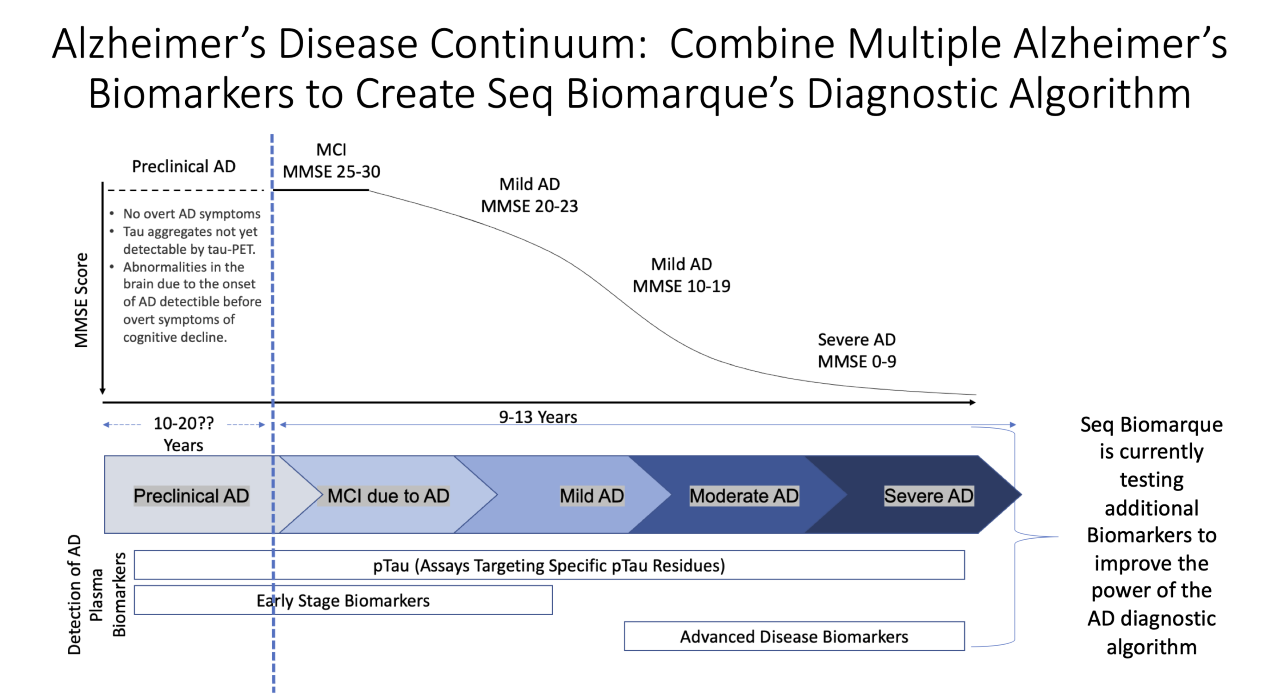
Seq Bio Marque is combining multiple Alzheimer’s Biomarkers to create a diagnostic algorithm that may identify patients with early-onset Alzheimer’s Disease.
Looking Beyond Tau: Seq Biomarque is Targeting Other Alzheimer’s-Related Biomarkers
- Combining multiple AD biomarkers may enhance diagnostic accuracy, provide insight into specific AD phenotypes, and provide insight into the pathologic changes that occur through the AD continuum.
- Seq Biomarque is currently developing a Biomarker panel that includes Beta-Amyloid, neurofilament light chain (NfL), and others.
- Seq Biomarque is evaluating biomarkers that are novel to the diagnostic AD continuum
- Seq Biomarque’s platform is expandable to allow for simultaneous high-throughput biomarker testing of large batches of independent samples.



Stay Connected: